Large-Volume Injection and Urinary Care Solutions

Introduction to High-Capacity Clinical Consumables
Healthcare delivery depends on a broad range of consumables that support both routine and specialized clinical procedures. Among these, large-volume injection devices and urinary drainage products play a crucial role in hospitals, surgical centers, emergency units, and long-term care facilities. Their importance is amplified in high-acuity environments where accuracy, material reliability, and patient safety are essential. This article provides a professional, in-depth analysis of two key product categories that support advanced clinical care, focusing on their applications, quality expectations, and value for institutional healthcare providers.
The Role of Specialized Consumables in Modern Healthcare
As healthcare systems evolve, clinical procedures have become more standardized and protocol-driven. This shift places increased emphasis on the reliability of consumables used repeatedly across departments. Large-volume injection devices support irrigation, aspiration, and enteral procedures, while urinary catheters are integral to perioperative care, critical care monitoring, and long-term patient management. Selecting the right products in these categories directly affects workflow efficiency, infection control, and patient comfort.
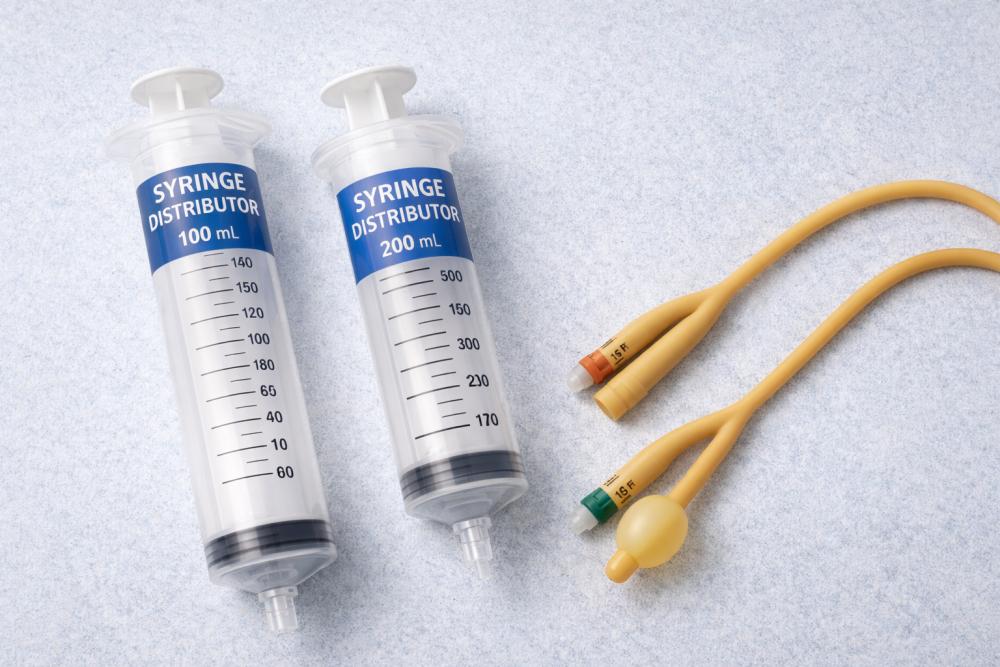
Large-Volume Syringes in Clinical Practice
Unlike standard injection devices, large-volume syringes are designed for applications that require controlled delivery or withdrawal of higher fluid volumes. These include wound irrigation, enteral feeding, suction procedures, and medication preparation in specific clinical scenarios. Their use spans multiple departments, including surgery, intensive care, urology, and emergency medicine. Because of their versatility, they must meet stringent performance and safety standards to ensure consistent outcomes.
Clinical and Procurement Value of Syringe distributor 100ml and 200ml
The availability of Syringe distributor 100ml and 200ml solutions is particularly relevant for healthcare institutions that require reliable access to high-capacity injection devices at scale. Syringes in these volume ranges are commonly used for bladder irrigation, aspiration procedures, and controlled fluid administration where precision and stability are critical. Their design must ensure smooth plunger movement, accurate volume markings, and structural integrity under repeated pressure.
From a procurement perspective, consistency across batches is essential. Healthcare providers rely on uniform performance to maintain standardized procedures and staff training. Large-volume syringes must also be manufactured from medical-grade materials that are compatible with a wide range of fluids and medications. Proper packaging and sterility assurance further support safe use in demanding clinical environments.
Impact on Workflow and Patient Safety
High-capacity syringes influence clinical workflows by reducing the need for repeated refilling during procedures. This efficiency minimizes procedural interruptions and lowers the risk of contamination. In critical care and surgical settings, where time and precision are paramount, such efficiency gains contribute directly to patient safety. Clear barrel graduation and ergonomic design also support accurate dosing and controlled handling by healthcare professionals.
Urinary Catheterization as a Core Clinical Procedure
Urinary catheterization is a common medical intervention used for bladder drainage, urine output monitoring, and postoperative care. Despite its routine nature, it carries inherent risks, particularly related to infection and patient discomfort. The choice of catheter material, design, and manufacturing quality significantly influences clinical outcomes. As a result, urinary catheters are subject to strict regulatory oversight and continuous evaluation by healthcare providers.
Clinical Significance of catheters foley natural rubber
Catheters foley natural rubber remain widely used in healthcare due to their flexibility, cost-effectiveness, and suitability for short- to medium-term catheterization. Their material properties allow for smooth insertion and effective bladder drainage when used according to clinical guidelines. In many hospital settings, these catheters are integrated into standardized care protocols for surgical recovery and acute urinary management.
From a clinical standpoint, proper balloon integrity, lumen consistency, and surface finish are essential to ensure safe performance. When manufactured under controlled conditions with medical-grade natural rubber, these catheters provide reliable functionality while supporting patient comfort. Clear labeling and size standardization further assist clinicians in selecting the appropriate device for individual patient needs.
Infection Control and Material Considerations
Infection prevention is a critical concern in urinary catheter use. Catheter-associated urinary tract infections remain a significant quality indicator in healthcare facilities worldwide. While no catheter eliminates risk entirely, material quality and manufacturing precision play an important role in minimizing complications. Smooth surfaces, consistent wall thickness, and reliable balloon inflation contribute to reduced trauma and improved drainage efficiency.
Healthcare institutions also evaluate compatibility with established infection control protocols. Single-use design, sterile packaging, and traceability are non-negotiable requirements. These factors support compliance with clinical guidelines and facilitate auditing and quality assurance processes within hospitals and care facilities.
Regulatory and Manufacturing Standards
Both large-volume syringes and urinary catheters are classified as medical devices and must comply with rigorous regulatory frameworks. These standards govern raw material selection, production processes, sterility validation, and post-market surveillance. Compliance with European and international medical device regulations provides assurance that products perform consistently and safely in clinical use.
Manufacturers serving professional healthcare markets are expected to operate certified quality management systems. Documentation, batch control, and ongoing performance monitoring are essential components of regulatory compliance. For healthcare providers and distributors, working with compliant manufacturers reduces legal risk and supports uninterrupted clinical operations.
Supply Chain Reliability in Institutional Healthcare
Consistent access to essential consumables is critical for healthcare continuity. Disruptions in the supply of large-volume syringes or urinary catheters can directly impact patient care and procedural scheduling. Healthcare procurement teams therefore prioritize suppliers with stable production capacity, predictable lead times, and transparent logistics processes.
Standardized packaging and clear product identification also support efficient inventory management. When consumables are easy to store, identify, and deploy, clinical teams can respond more effectively to both routine demand and emergency situations.
Procurement Decision-Making in Professional Markets
Procurement in healthcare increasingly emphasizes long-term value rather than short-term pricing. Decision-makers assess product performance, regulatory compliance, documentation quality, and supplier reliability. Products that integrate smoothly into existing clinical protocols reduce training requirements and operational complexity.
ROMED HOLLAND operates within this professional healthcare context, supplying medical consumables designed to meet the expectations of hospitals, distributors, and institutional buyers. Their focus on quality-controlled manufacturing and regulatory alignment supports healthcare organizations seeking dependable solutions for high-volume clinical use.
Contribution to Patient Outcomes and Clinical Confidence
Reliable consumables contribute directly to better patient outcomes by supporting safe, efficient procedures. Large-volume syringes enable controlled fluid management, while well-manufactured urinary catheters support effective drainage and monitoring. Together, these products reduce procedural risks and enhance clinician confidence.
Patients benefit from smoother procedures, reduced discomfort, and lower complication rates. Although often unseen by patients, the quality of these devices plays a vital role in shaping their overall care experience and trust in healthcare providers.
Conclusion
High-capacity injection devices and urinary catheters are integral to modern clinical practice. Their performance, safety, and availability influence daily operations across a wide range of healthcare settings. By prioritizing regulated manufacturing, material quality, and supply chain reliability, healthcare organizations can strengthen care delivery and maintain high clinical standards. Strategic investment in trusted medical consumables ensures that both patients and professionals are supported in delivering effective, safe, and sustainable healthcare services.
